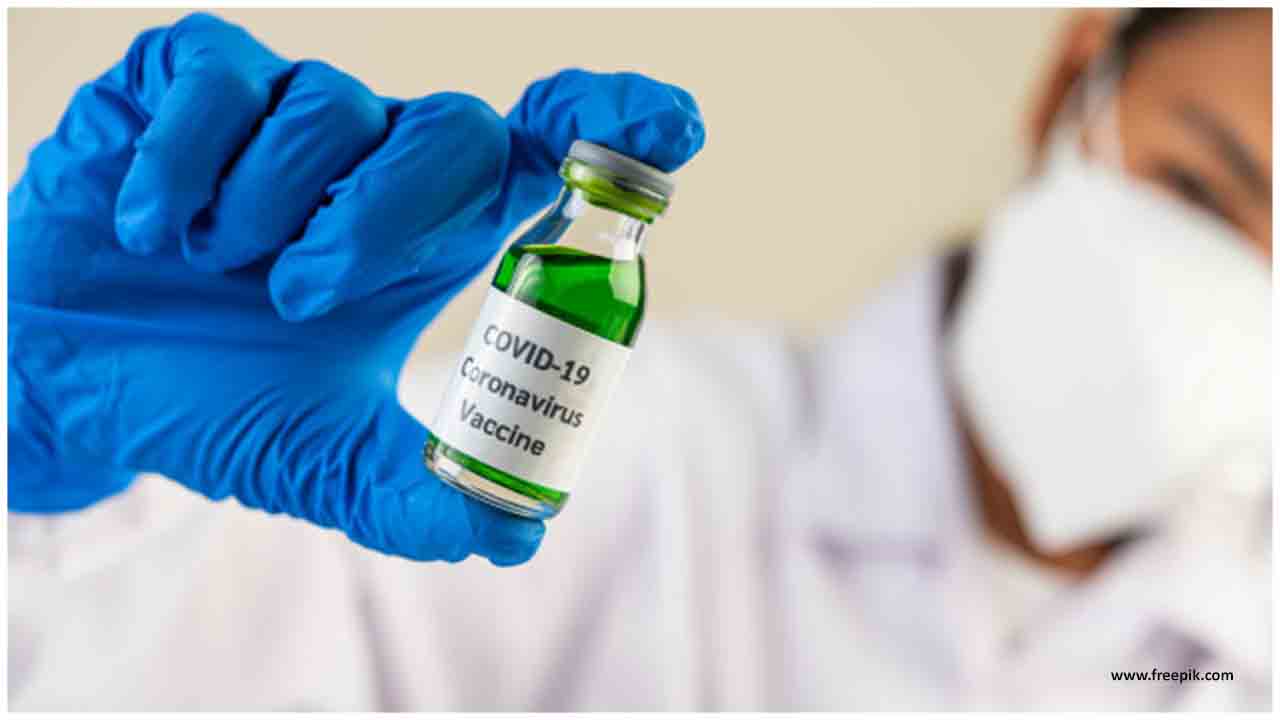
Stage III clinical preliminaries of a COVID-19 immunization in Abu Dhabi utilizing around 15,000 volunteers, the legislature in the capital of the United Arab Emirates said on Thursday.
The human preliminary is an association between Sinopharm's China National Biotec Group (CNBG), Abu Dhabi-based man-made consciousness and distributed computing organization Group 42 (G42) and the Abu Dhabi Department of Health.
The examination, which started on Wednesday, is the world's first Phase III preliminary of an inactivated immunization, G42 Healthcare CEO Ashish Koshy said. Inactivated immunizations are notable and have been utilized against infections, for example, flu and measles.
No COVID-19 immunization has yet been endorsed for business use. As per a WHO rundown of the condition of antibody improvement for COVID-19, there are 23 expected immunizations in human preliminaries, with three of them in or beginning huge scope late-stage, or Phase III, preliminaries to test adequacy.
The preliminary will test two antibody strains and fake treatment. Two portions three weeks separated will be regulated and chips in followed for a year, said Nawal Alkaabi, top of the UAE's COVID-19 Clinical Management Committee.
Around 15,000 volunteers more than three to a half year will be selected, at first in Abu Dhabi. They will be 18 to 60 years old with no genuine fundamental clinical issues and without past COVID-19 contamination, Alkaabi said.
Sinopharm picked the United Arab Emirates in light of the fact that around 200 distinct nationalities dwell there and it has attention on clinical exploration and battling the pandemic, Koshy said.
The UAE says it has led in excess of 4 million coronavirus contamination tests on a populace of around 9.6 million. It has recorded very nearly 56,000 instances of disease and 335 passings.
Sinopharm made sure about endorsement for the preliminary in late June. The test immunization passed Phases I and II of clinical preliminaries with 100% of volunteers creating antibodies following two portions in 28 days, an Abu Dhabi government proclamation said.
China has been looking abroad to preliminary potential antibodies as a result of the absence of new patients at home. China's Sinovac Biotech is directing Phase III preliminaries of an antibody in Brazil.
Sinopharm and G42 would not approach understanding information in the preliminary which would be led in Abu Dhabi state emergency clinics, G42 Healthcare research chief Walid Zaher stated, adding the UAE expected to produce any subsequent effective immunization.
G42 is an Abu Dhabi-based man-made consciousness firm that has joined forces with Chinese genomics organization BGI to assemble a COVID-19 testing research facility in the emirate and with Israeli contractual workers to create innovations to help battle the ailment.
Koshy said the organization was exclusive however declined to state by whom.
Like other Gulf expresses the UAE has grown close binds with China, looking for capital and innovation to differentiate its economy away from hydrocarbon incomes.
Nonetheless, key partners, the United States has cautioned Gulf states to continue with alert and to think about their relationship with Washington.
 The human trial is a partnership between Sinopharm's China National Biotec Group (CNBG), Abu Dhabi-based artificial intelligence and cloud computing
The human trial is a partnership between Sinopharm's China National Biotec Group (CNBG), Abu Dhabi-based artificial intelligence and cloud computing 
























.jpg)





