The primary solid volunteer has now gotten a coronavirus antibody created by Imperial College London scientists, the group behind the task said on Tuesday.
The clinical group, who conveyed a little portion of the immunization to the member at a west London office, are intently checking the member and report they are healthy, with no security concerns.
The human preliminaries of the immunization are likewise the principal trial of another self-enhancing RNA (saRNA) innovation.
"We have arrived at a huge achievement in this momentous examination with the primary portion of a self-enhancing RNA immunization conveyed securely," said Dr. Katrina Pollock, from Imperial's Department of Infectious Disease and Chief Investigator of the examination.
"We are presently ready to test the antibody in the portion assessment stage before pushing ahead to assessing it in bigger numbers," she stated, including that thousands have been joining to be a piece of the continuous immunization contemplates.
Royal College London's immunization up-and-comer is being created and trialed as a feature of 41 million pounds in subsidizing from the UK government and a further 5 million pounds in generous gifts.
The group says that it has experienced "thorough" pre-clinical wellbeing tests and in creature contemplates it has been demonstrated to be protected and delivered empowering indications of a successful resistant reaction.
The new saRNA innovation being trialed with it is professed to can possibly reform antibody advancement and empower researchers to react all the more rapidly to rising maladies.
"The main member denotes a significant advance for our saRNA immunization stage, which has at no other time been trialed in people," Professor Robin Shattock, from the Department of Infectious Disease at Imperial College London, who is driving the work.
"We currently excitedly anticipate quick enlistment to the preliminary so we can evaluate both the security of the immunization and its capacity to create killing antibodies which would show a successful reaction against COVID-19," he said.
The primary volunteer, who has requested to stay unknown, has now gotten a first portion antibody, with a subsequent supporter portion to follow inside about a month. A few others are relied upon to get the first portion over the coming days.
The clinical group will keep on observing all members intently for security, just as hoping to check whether they produce antibodies against the SARS-CoV-2, or COVID-19, infection.
In the underlying phase of the preliminary, 15 solid volunteers are getting the immunization - beginning with a low portion and raising to progressively higher dosages for ensuing volunteers - to survey security and to locate the ideal measurements. Over the coming weeks, 300 sound members are required to get two portions of the antibody.
On the off chance that the immunization is protected and shows a promising insusceptible reaction in people, at that point bigger preliminaries would be made arrangements for later in the year.
The Imperial College London immunization is the UK's second significant antibody competitor close by one being tried by Oxford University, additionally upheld by the UK government through its continuous human preliminaries.
The UK has announced 305,289 diseases of coronavirus up until this point and the passings remain at 42,647.
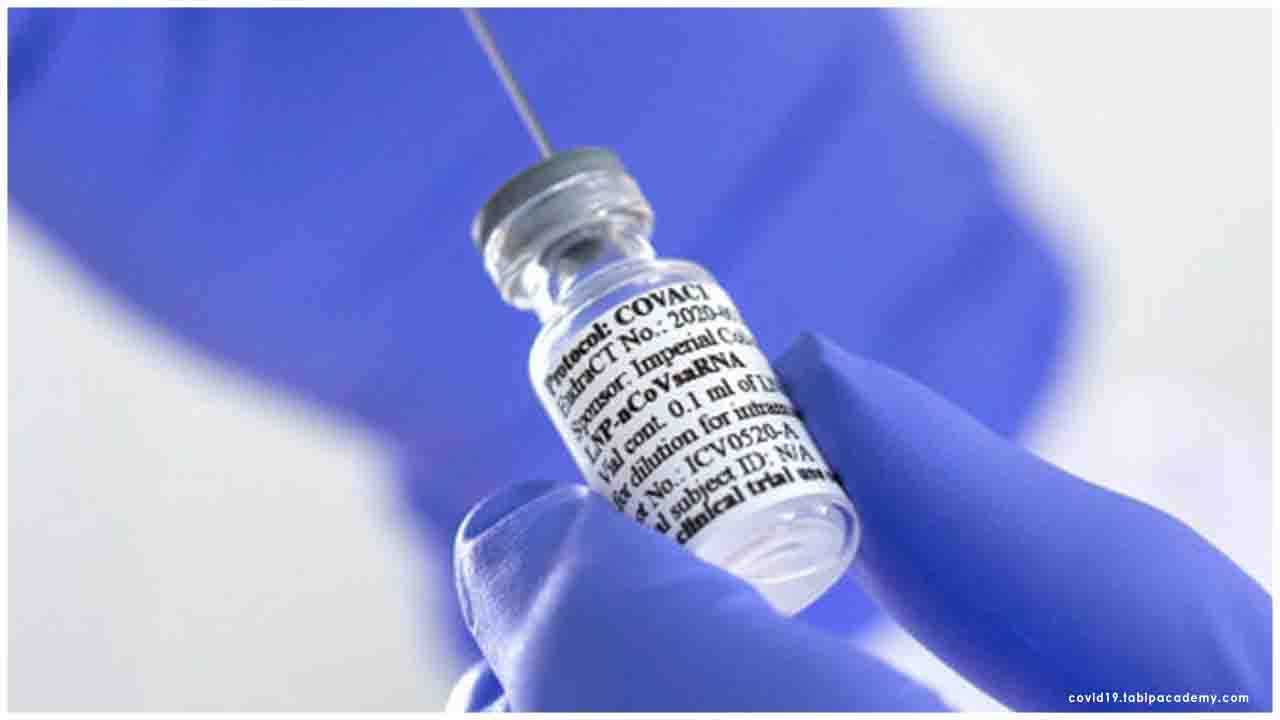
 The team, who delivered a small dose of the vaccine to the participant at the London facility, are monitoring the participant and report they are in good health
The team, who delivered a small dose of the vaccine to the participant at the London facility, are monitoring the participant and report they are in good health 
























.jpg)





