Reistone Biopharma Company Limited (Reistone), a clinical-stage biopharmaceutical company focused on developing novel medicines for autoimmune and inflammatory diseases, today announced dosing of the first patient in their Phase II, global clinical trial (RSJ10521) of SHR0302, a potent and highly selective small-molecule inhibitor of Janus kinase type 1 (JAK1) in patients with moderate and severe Alopecia Areata. This clinical study is running in China, the United States and Australia.
Alopecia Areata is one of the common dermatological diseases, with increasing scientific evidence on involving in dysfunction of human body immune system, where body T cells attack the hair follicles resulting in transient non-scarring hair loss. The hair loss may last for a few weeks to decades or can be permanent. Globally, about 2% of the population will be affected by this disease in their lifetime. Unfortunately, there is no treatment approved by regulatory agencies for Alopecia Areata so far. Unmet needs remain high for Alopecia Areata treatment with a growing clinical evidence proving that JAK inhibitors are potential treatments.
"One of the most common treatments is corticosteroid either administered orally or as an injection. Oral corticosteroid has its side-effect while multiple injections to the scalp can cause significant pain, and is not acceptable to many," stated Dr. Min Irwin, Chief Executive Officer of Reistone, "We are very excited about the study and dosing the first subject, which represents another key milestone for Reistone as it marks the second phase II program in dermatology therapy area for this compound."
Aik Han Goh, M.D., Chief Medical Officer of Reistone, noted, "Given that JAK-STAT pathway plays an important role in CD8+ T cell-mediated Alopecia Areata pathogenesis, SHR0302, an oral JAK inhibitor has great potential to be an alternative treatment for Alopecia Areata. The impact of Alopecia Areata goes far beyond hair loss. It causes not only physical, but emotional and social discomfort, including social isolation, embarrassment, and in severe cases leading to anxiety and depression. We are devoted to improving the quality of life in patients with Alopecia Areata."
SHR0302 is a novel, potent, orally administered selective Janus kinase type 1 (JAK1) inhibitor in development as a treatment for inflammatory bowel diseases. JAK1 selectivity could potentially provide a favorable safety and efficacy profile compared to the pan-JAK inhibitor. Longer-term clinical studies are ongoing to confirm a favorable risk-benefit of JAK1 selectivity by avoiding the hematological side effects related to JAK2 inhibition. Reistone licensed in the drug from Jiangsu Hengrui Medicine Co., Ltd and owns the global rights for multiple indications of autoimmune diseases.
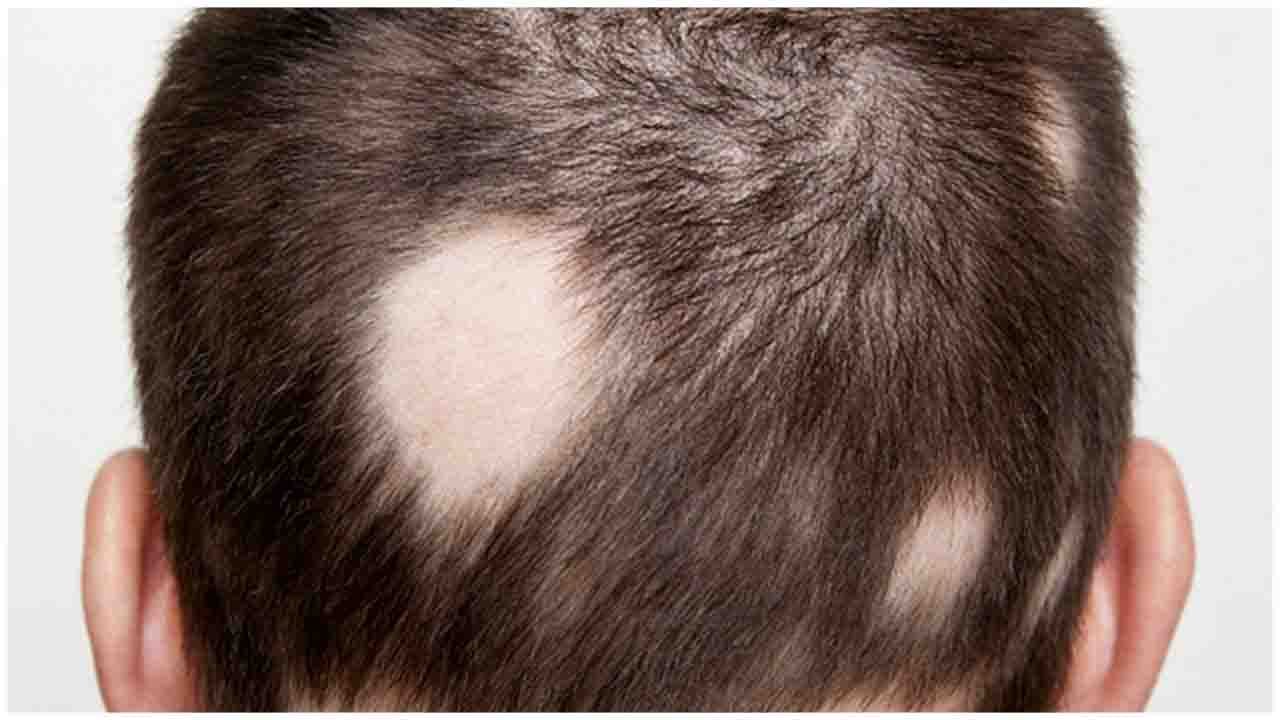
 A cure for baldness may be in the offing as phase 2 trial starts globally
A cure for baldness may be in the offing as phase 2 trial starts globally