1. Introduction: New Medical Device Rule and Deadline
Earlier this year The Indian medical device landscape underwent a significant transformation with the implementation of the Medical Device Rules (MDR) , which order a deadline of October 1. This comprehensive regulatory framework introduced a risk-based classification system for medical devices, categorizing them into Class, C, and D based on their potential risk to patients.
2. Categorizing Risk: Understanding the New Deadline by CDSCO
According to the new regulations by the Central Drugs Standard Organization (CDSCO), medical devices like ventilators, imagining equipment, oxygen therapy equipment, nebulizers, x-ray equipment, surgical robots and oncology treatment linear accelerators, can't be sold in the market without proper manufacturing licensed from October 1.
This approach allows for tailored regulatory requirements corresponding to the risk level, ensuring a more efficient and targeted regulatory process.
3. Delayed Process: High-Risk Device Manufacturers
While the risk-based classification system aims to improve patient safety, many high-risk medical device manufacturers face challenges due to these regulatory changes.
Many manufacturers express that have filed for manufacturing licenses since July but we're still waiting to get the updates and audits on their production devices.
However, a manufacturer said, "We are still unaware of the further process regarding the licensing process and no updates issued from the health ministry or drugs regulatory on this licensing service and thus we've stopped manufacturing and also we cannot manufacture further from October 1." This pause in production will lead to a shortage of essential medical devices, impacting patients and healthcare staff.
4. Impact on Patients and Healthcare Providers
The shortage of critical medical devices has resulted in delays in treatment and increased healthcare costs for patients and healthcare providers. Patients requiring high-risk medical devices are particularly affected, as access to these devices is crucial for their health and well-being. The delays in obtaining these devices can be a matter of life and death in some cases, underscoring the urgent need to address the challenges faced by manufacturers.
5. Medical device shortage and Delays in granting licenses
Rajiv Nath the coordinator of the Medical Devices Association for India has indicated the potential effects of a shortage in the production of medical devices supply chain in the coming future. He highlighted the current shortage of moderately high-risk medical devices classified into Class C and Class D. This shortage happened due to the delay in issuing manufacturing licenses by the health advisory. He further added that the manufacturing license has not been granted to even those manufacturers who have already registered and applied for this License.
6. Conclusion: The road ahead for the Indian Medical Device Industry
As the Indian medical device industry adapts to the new regulations from October 1, a balanced approach, taking into account the needs of patients, the healthcare system, and manufacturers, will be helpful in shaping a sustainable medical device industry. The future lies in effective collaboration and thoughtful policy adjustments to ensure both patient safety and industry growth
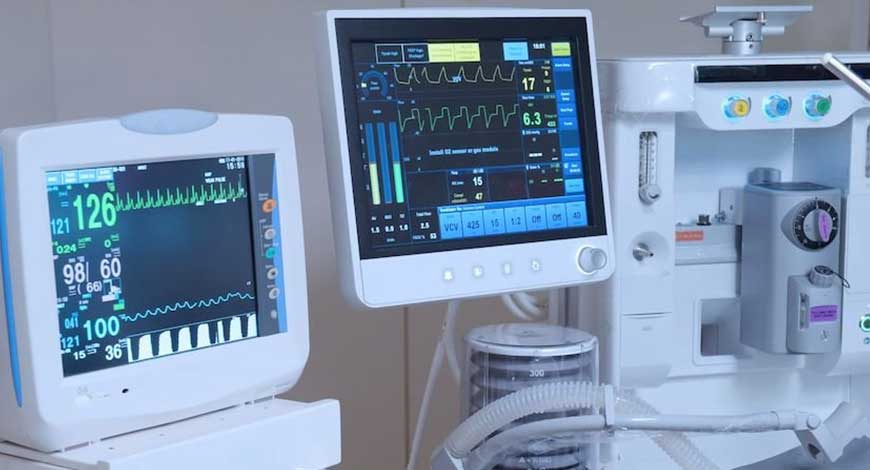
 Medical devices Manufacturers have a new guideline, The Central Drugs Standard Organization (CDSCO) issued a new deadline of October 1, for the categorized medical devices under Class C And Class D, to have a proper manufacturing license, for quality control. The essential medical devices like ventilators, oxygen therapy equipment, surgical robots and cancer treatment Linear accelerators can't be sold in the market without a manufacturing license from October 1.
Medical devices Manufacturers have a new guideline, The Central Drugs Standard Organization (CDSCO) issued a new deadline of October 1, for the categorized medical devices under Class C And Class D, to have a proper manufacturing license, for quality control. The essential medical devices like ventilators, oxygen therapy equipment, surgical robots and cancer treatment Linear accelerators can't be sold in the market without a manufacturing license from October 1.














.jpg)












_elected_as_global_president_of_the_world_congress_of_ophthalmic_anaesthesia.jpg)