Enzymatica announces today the preliminary results of an in vitro study showing the ability of the mouth spray ColdZyme to deactivate SARS-CoV-2, the virus causing the COVID-19 pandemic. The study demonstrated that ColdZyme deactivates SARS-CoV-2 coronavirus by 98.3% (1.76 log10). The results indicate that ColdZyme can offer a protective barrier against harmful viruses such as SARS-CoV-2 by local virus deactivation in the oral cavity.
The medical device ColdZyme is a mouth spray that forms a barrier in the oral cavity against common cold viruses. The barrier solution of the device is mainly composed of glycerol and Atlantic cod trypsin. The goal of the present study was to determine the ability of ColdZyme to deactivate SARS-CoV-2 known to cause the COVID-19 pandemic.
A virucidal efficacy suspension test was conducted using ColdZyme against SARS-CoV-2. ColdZyme deactivated SARS-CoV-2 by 98.3% (1.76 log10) in 20 minutes. Furthermore, no cytotoxicity was detected for ColdZyme at any dilution tested. The study was conducted by the US company Microbac Laboratories Inc - an independent, accredited and certified laboratory.
The in vitro study was based on a standardized and validated methodology, i.e. ASTM International test method designated E1052 "Standard Test Method to Assess the Activity of Microbicides against Viruses in Suspension".
Previous in vitro results made with the same method showed that ColdZyme is effective against another Coronavirus, HCoV-229E, one of the causes of the common cold, and in comparison to SARS-CoV-2 belongs to another subgroup within the corona family. The aggregated results indicate that ColdZyme can be effective against a variety of Coronaviruses.
SARS-CoV-2 actively replicates in the throat and shows high viral shedding also at a time of mild symptoms. Therefore, ColdZyme sprayed onto the mouth and throat could lower the risk of infection, and decrease the viral load locally. The lowered viral load may decrease viral shedding and thus minimize the spread of SARS-CoV-2.
"Even if the current in vitro results cannot be directly translated into clinical efficacy, it is very interesting that ColdZyme is able to effectively deactivate SARS-CoV-2 in vitro since it constitutes a proof-of-principle that can be taken further into clinical studies. Thus, the results indicate that ColdZyme can offer a protective barrier against SARS-CoV-2," says Claus Egstrand, Enzymatica's Chief Operating Officer.
Enzymatica AB is a Swedish life science company that develops and sells health care products for primary conditions of the ear-nose-and-throat region. The products are based on a barrier technology that includes marine enzymes. The company's first product is the medical device ColdZyme, a mouth spray against the common cold. The product has been launched in about ten markets. The strategy is to continue to grow by developing more health care products and strengthening the company's position in existing markets and expanding into new geographic markets through established partners.
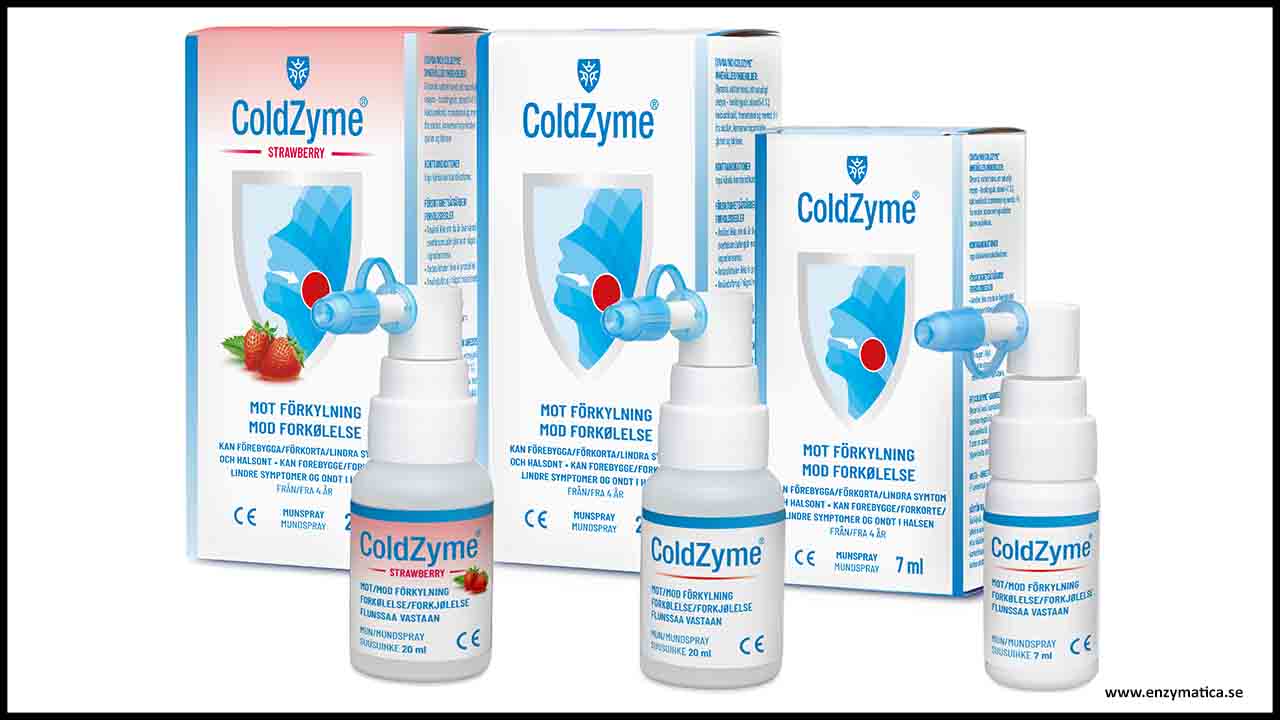
 Swedish company claims nasal spray ColdZyme could deactivate COVID-19 causing virus in mouth
Swedish company claims nasal spray ColdZyme could deactivate COVID-19 causing virus in mouth




















.jpg)












