The United States on Tuesday consented to a $1.95 billion arrangement with US pharma mammoth Pfizer and Germany's BioNTech for 100 million dosages of their test COVID-19 immunization, some portion of a forceful push to begin inoculating Americans right on time one year from now.
It is the greatest arrangement to date under Operation Warp Speed, expected to quicken the turn of events, assembling, and circulation of coronavirus immunizations, therapeutics, and diagnostics.
Pfizer and BioNTech, which are building up the medication together, said in articulations that the American individuals would get the future immunization "for nothing" following the Trump organization's vow.
Under the understanding, the US government has put in an underlying request for 100 million portions to be conveyed if the administrative endorsement is conceded.
The US government likewise has a choice to buy upwards of 500 million extra dosages from the two firms.
BioNTech and Pfizer have limited their antibody up-and-comers down to two leaders and are trusting that the green light will start a mass preliminary including 30,000 solid volunteers, which may happen not long from now.
If the investigations are fruitful, they hope to get some type of crisis endorsement as right on time as of October 2020.
Not long ago, they declared that early outcomes demonstrated their lead applicant created killing antibodies in people at or over the levels saw in recouped COVID-19 patients.
This was practiced with generally low dosages and caused symptoms that were mellow to direct however transient, which is viewed as typical.
"We are gathering an arrangement of antibodies to expand the chances that the American individuals will have at any rate one protected, viable immunization when the finish of this current year," said wellbeing Secretary Alex Azar of the arrangement.
"We are regarded to be a piece of this push to give Americans access to assurance from this lethal infection," included Albert Bourla, director and CEO of Pfizer.
- Vaccine race -
Labs around the globe are dashing to deliver an immunization to help end the most noticeably terrible wellbeing emergency in longer than a century.
More than 200 applicant immunizations are presently being created with approximately two dozen at the phase of clinical preliminaries with human volunteers.
Not long ago, the US marked a $1.6 billion arrangement with Novavax for 100 million dosages.
In May, the administration declared up to $1.2 billion for AstraZeneca's competitor antibody, created related to the University of Oxford.
The US has additionally declared $456 million for Johnson and Johnson's antibody up-and-comer; $486 million for Moderna's; and $628 million for Emergent Biosolutions.
The administration is similarly putting resources into assembling limits at its hazard and burning through several million in organizations that produce needles, vials, and clinical glass-covered plastic compartments.
- RNA antibody -
The Pfizer-BioNTech antibody strategy depends on utilizing flag-bearer RNA, a hereditary code from the SARS-CoV-2 that slips into human cells to create an engineered type of the infection's spike protein.
This thus makes the host create antibodies. The thought behind the innovation is decades-old, yet has never carried an immunization to administrative endorsement.
A BioNTech representative disclosed to AFP that two infusions would likely be required for greatest insurance, with the supporter shot after seven days after the main infusion.
Given the cost paid by the US government, it would thusly cost $39 to vaccinate an individual against the dangerous infection.
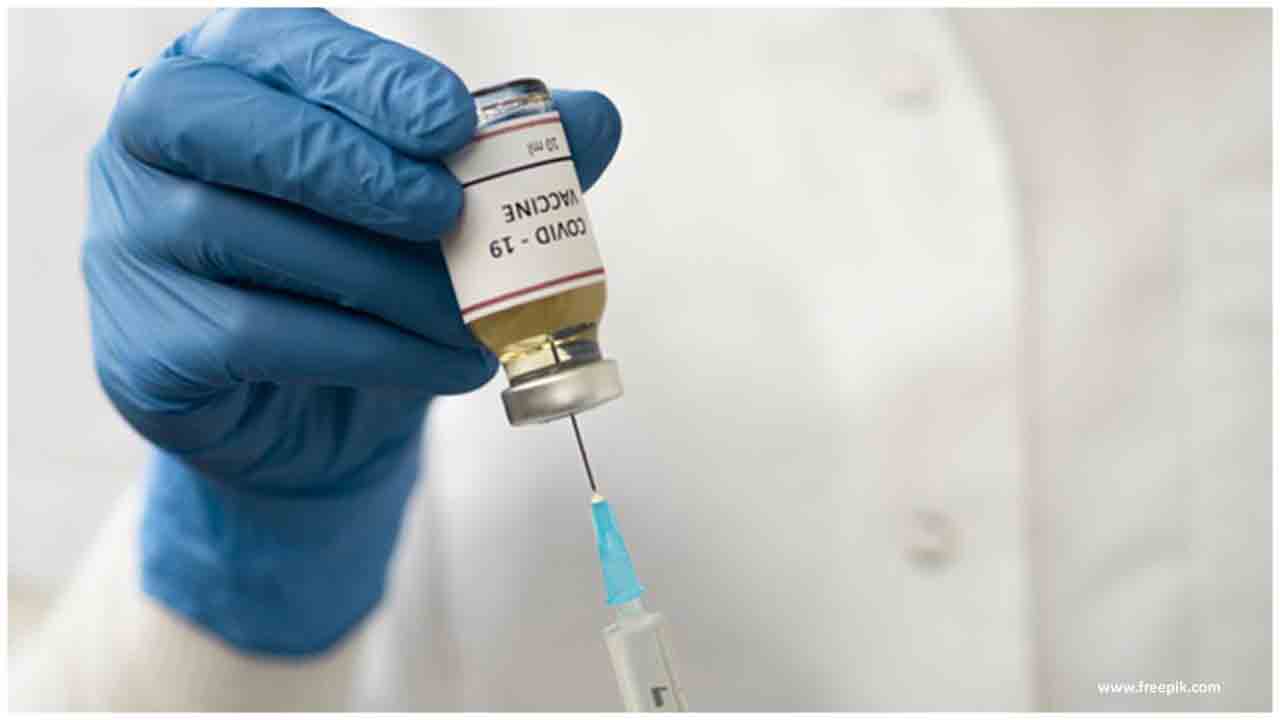
 Pfizer and BioNTech, which are developing the drug together, said in statements that the American people would receive the future vaccine "for free"
Pfizer and BioNTech, which are developing the drug together, said in statements that the American people would receive the future vaccine "for free"




















.jpg)











